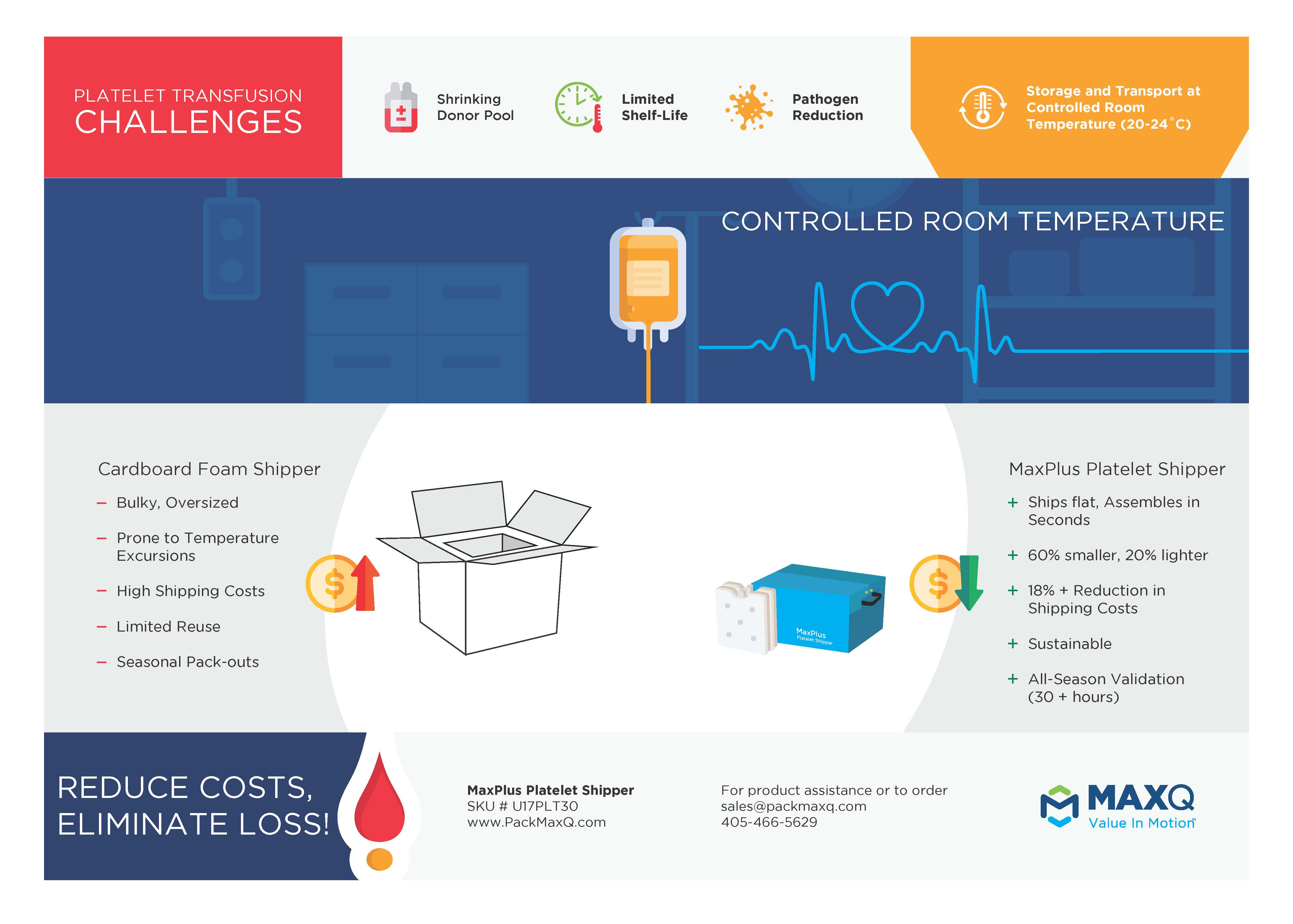
How to Comply with FDA Guidance on Bacterial Risk Controls for Platelets
Cerus’ INTERCEPT® blood systems are the front-runners in pathogen-reduced platelet storage. Proper conduct for shipping INTERCEPT platelet bags is the following**: “Inspect the final storage containers for any scrapes or leaks. If a leak is detected, follow instructions in General Product Handling. Routinely disinfect shipping containers per institutional procedure and schedule. Remove clamps from containers […]