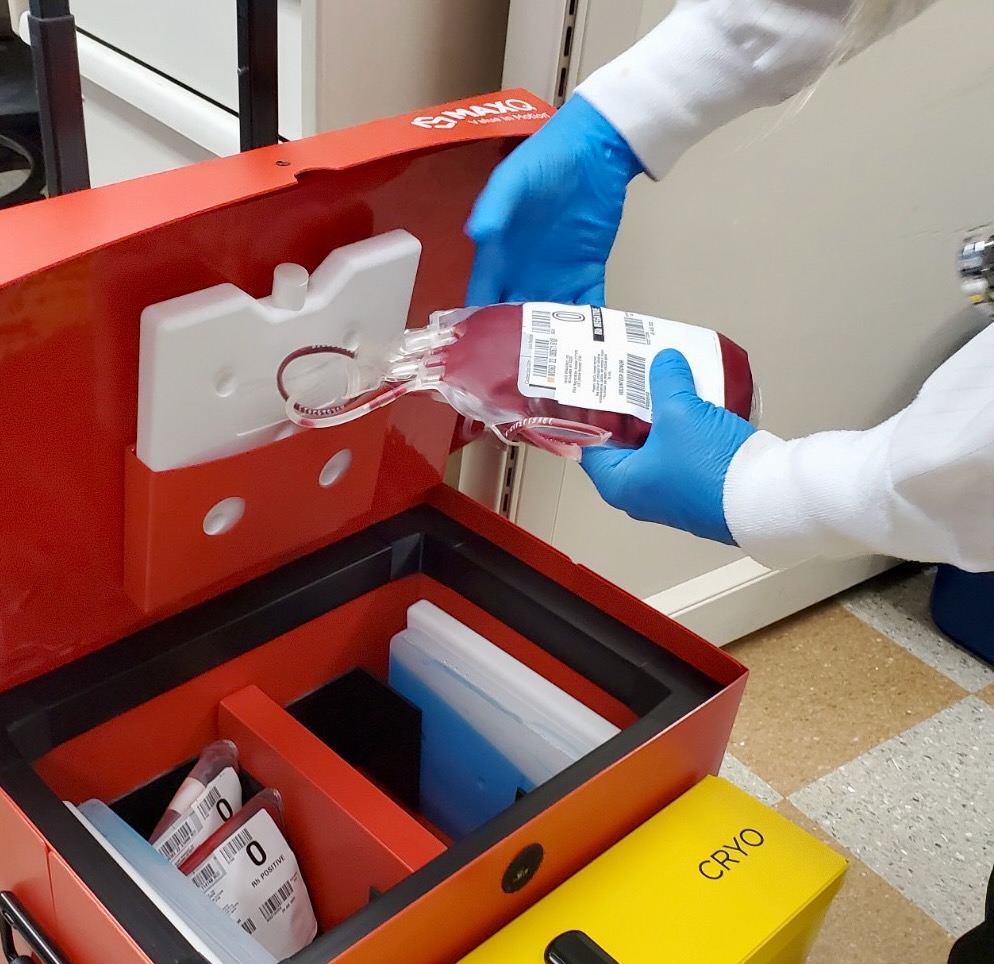
Using MaxPlus MTP Cooler® to Standardize Processes Across Blood Banks
Learn how a network of 10 Upper Midwest hospitals came together in 2019, it became clear pretty quickly to the organization’s Manager for Transfusion Services there was a lack of consistent processes across the blood banks. That’s why streamlining how blood products were moved around the hospital and transported to other sites was a priority from the outset. Standardizing packout processes was essential to making that happen – and the place to start was with the coolers.