Are you ready for Pathogen Reduced (PR) Platelets?
Updated FDA guidelines and recommendations for controlling risk of bacterial contamination require shipping and distribution innovations, like MaxQ’s PR Platelet Shipper.

Updated FDA guidelines and recommendations for controlling risk of bacterial contamination require shipping and distribution innovations, like MaxQ’s PR Platelet Shipper.

Shipping and distribution of blood and blood products can be challenging and are complicated by diverse operational needs and compliance requirements. Max Q is responding…

Multi-Product MTP Cooler helps improve response time and decrease blood product waste MaxPlus Massive Transfusion Protocol Coolers are designed to accommodate all MTP needs. They store 1-6 units of red blood cells, 1-6 units of thawed plasma (warm or chilled) and 1-2 platelet units at optimal temperatures for up to 12 hours. Introducing the new […]

Shipping plasma requires a hefty amount of dry ice per shipment, resulting in bulk packaging and higher costs, involving hazards and specialized training. MaxQ has an “ice free” solution.
Expansion includes state of the art upgrades to our testing lab, and addition of new test equipment and software at our NIST traceable cold chain package testing lab for increased capacity.

MaxQ presented its innovation on dynamic energy response for cold chain shipments using its patented MaxTrace™ technology.
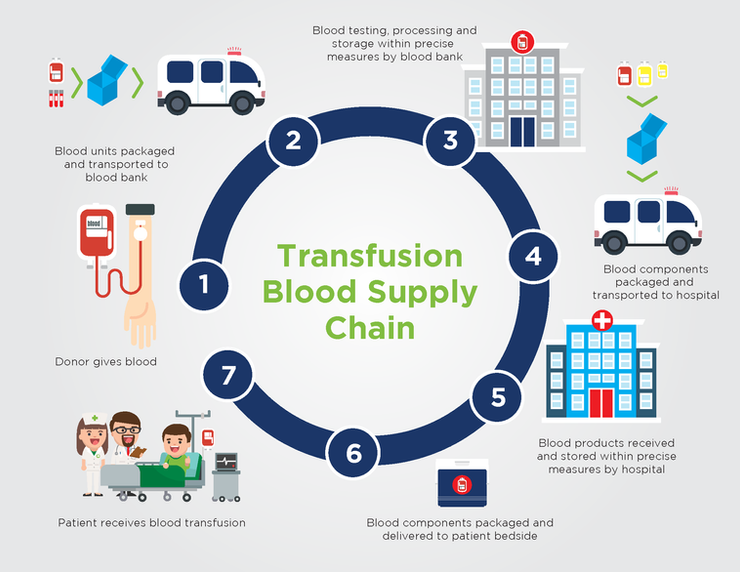
Blood products must go through an elaborate series of steps before they are transfused into the patient. This is known as the blood supply chain, which is illustrated in this infographic.

Learn more about the quality, compliance and cost issues that can be inherent with traditional blood transport methods.
Testing custom footer content area in maxq/footer.php file