Validation and Qualification Insights
In this Max Q & A, Senior Solutions Architect Tyler Rapp shares his experiences and knowledge about validations – common mistakes, observations and tips as well as how MaxQ can help make the process easier.

In this Max Q & A, Senior Solutions Architect Tyler Rapp shares his experiences and knowledge about validations – common mistakes, observations and tips as well as how MaxQ can help make the process easier.

The trend toward direct-to-patient specialty medication delivery has been growing for several years, which has only been accelerated by COVID-19 restrictions. These medications are often biologics and must be kept within strict temperature ranges. For specialty pharmacies this has meant purchasing and storing bulky, expensive, and single-use packaging solutions – cumbersome for both pharmacists and patients – and incurring increasing inbound and outbound freight costs, as well as negative environmental impact.

Recent updates to USP’s COVID-19 Vaccine Handling Toolkit address a number of important operational considerations around handling, storage and transport.

What is AABB’s Standards-Compliant Product Program? AABB’s Standards Compliant Products Program was launched in late 2019 to support vendors and manufacturers who provide equipment and supplies for transfusion services or blood collection. Upon applying, the specific product is reviewed thoroughly by AABB’s Accreditation Department members before being approved as an “AABB Standards-Compliant Product”. Why is […]

Welcome to MaxQ&A – designed to bring you insights from experts about what’s happening in the life sciences and blood industry.

MaxQ Research is pleased to announce the hiring of Jeffrey Gutkind as Director of Sales Life Science Packaging. Gutkind will manage the sales, marketing, and service strategies….

MaxQ has been awarded a Phase I SBIR contract from the United States Defense Health Agency (DHA) SBIR/STTR Program to help address the challenges faced by the U.S. Armed Services Blood Program (ASBP)

Cerus’ INTERCEPT® blood systems are the front-runners in pathogen-reduced platelet storage. Proper conduct for shipping INTERCEPT platelet bags is the following**: “Inspect the final storage containers for any scrapes or leaks. If a leak is detected, follow instructions in General Product Handling. Routinely disinfect shipping containers per institutional procedure and schedule. Remove clamps from containers […]

Cardboard boxes as shipping containers for biological substances are outdated. They may be light, but they are not particularly durable. Wear quickly appears after just one shipment as it transfers hands in the supply chain. Sticker removal can damage the box integrity, as can removing the tape when opening the package. Cleaning the boxes for […]
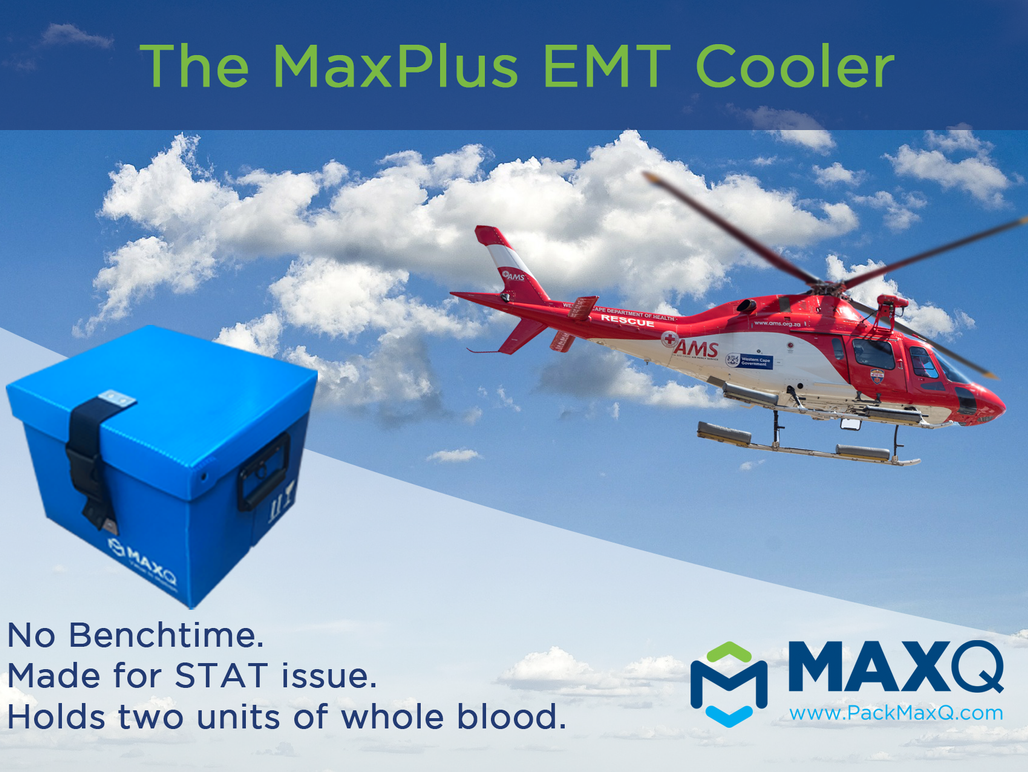
Evolving needs for STAT issue of pre-hospital blood transfusion presses need for qualified coolers that are optimized for emergency transport. MaxQ has responded with its MaxPlus EMT Cooler.
Testing custom footer content area in maxq/footer.php file